Take a Guided Tour of CO2 Extraction: Part 1
From the machinery and components through to solvent recovery, learn how CO2 extraction is made possible!
PART 1 — GET FAMILIAR WITH THE SYSTEMS AND EQUIPMENT INVOLVED, FROM PUMP TECHNOLOGY TO PHASE MANAGEMENT.
Carbon dioxide (CO2) makes an excellent extraction solvent for botanical oils. CO2 is a unique solvent as it retains its solvency power as either a (subcritical) liquid or as a supercritical fluid depending on the respective temperature and pressure. By changing the pressure and temperature of CO2, its solubility and selectivity for a specific compound of interest can be changed to optimize an extraction.
This three-part series will provide a guided tour through the process of extraction using CO2 as the extraction solvent. Various aspects of the extraction system will be covered ranging from the machinery and components, the different parameters that can be used, to the interwoven principles of extraction (see Figure 1 for an overview). The first part will provide a mechanical focus on the early stages of the process, particularly on storage of the solvent and the distribution of the solvent via a dual acting positive displacement pump. Part two will examine what occurs during the extraction process in the extraction chamber, solvent power and the associated solubility. Finally, part three will cover the separation of the solutes from the solvent stream and solvent recovery.
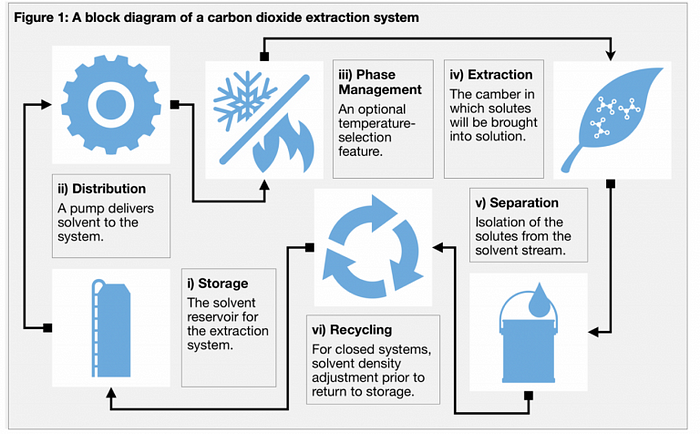
1. STORAGE
The extraction process begins with the CO2 accumulator. This is the reservoir that supplies the system with solvent during operation. CO2 can be stored here as either a low-pressure gas, a high-pressure gas or a liquid.
2. DISTRIBUTION
The pump is the next stage of the process. The job of the pump is to deliver CO2 to the system at a selected pressure. The two most common pumps that are used in the extraction industry today are dual acting positive displacement pumps and diaphragm pumps.
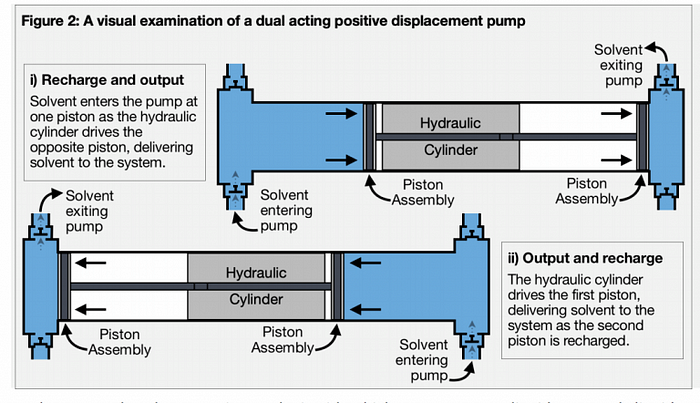
DUAL ACTING POSITIVE ACTING DISPLACEMENT PUMPS
Dual acting positive displacement pumps have the ability to deliver an uninterrupted flow of solvent into the extraction system. In turn, the pump’s hydraulic cylinder applies force to two oppositely directed pistons. Liquid enters the available space ahead of one piston, as force is applied to the other to deliver a volume of the solvent. At the completion of this stroke, force is then applied to the opposite piston, now primed with a volume of solvent ready for delivery to the next section of the machine. Hence, dual acting positive displacement pumps eliminate the interruption in solvent output (by eliminating the down stroke). Figure 2 shows the recharge and output operation of the dual acting positive displacement pump. Due to their efficiency and continuous solvent delivery, and the fact that their design is very robust, they are the favored option for use in extraction equipment.
DIAPHRAGM PUMPS
Briefly, solvent only enters diaphragm pumps on their down stroke and is then delivered on their output stroke (Figure 3). Despite numerous variations on their designs, solvent delivery results from these pulses; thus, the system will experience an interruption in the flow of solvent at each down stroke as the pump is primed with a new volume of solvent for delivery. Furthermore, diaphragm pumps generally have smaller displacements (being that the pump strokes provide a lesser fluctuation in internal volume) and operate at higher frequencies (more cycles for the life of the operation) which results in increased wear and system pulsation. This style of pump is also known to be less robust, making it less reliable, which leads to potential increased downtime for maintenance and component failure.

Regardless of what pump is chosen, as the CO2 reaches the pump, it must either be as high-pressure gas or in a liquid state. As previously mentioned, only liquid and supercritical phases of CO2 have adequate solvent power to be used in extraction. It is important to note that if a high-pressure gas is delivered to the chamber, enough additional pressure must be built up within to produce a liquid or supercritical fluid. If the solvent is pumped as a liquid, no change of phase is required. However, an operator may wish to adjust the fluid temperature which would include potential selection of the supercritical phase, before the solvent reaches the extraction chamber.
PHASE AND EFFICIENCY
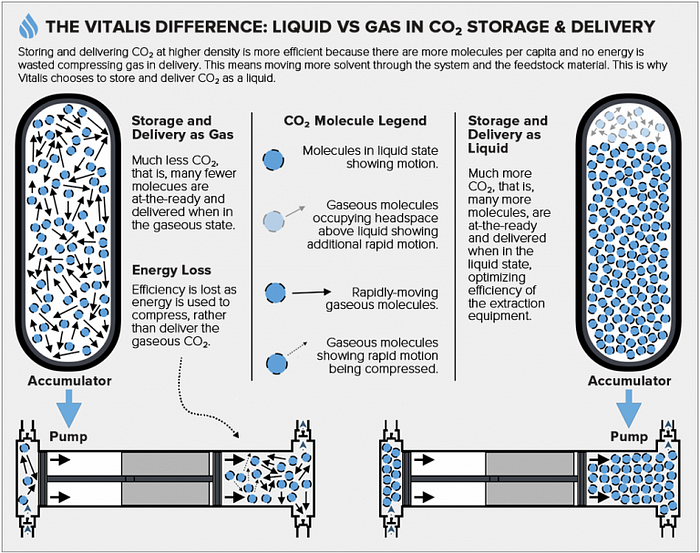
Importantly, the phase of the solvent as it is acted upon by a system’s solvent-delivery pump can affect the extraction machinery’s mechanical efficiency. Liquids are effectively non-compressible, meaning the force applied by the pump is used to efficiently deliver solvent to the system. Conversely, more work is required when applied to a volume of gas and this will be given off as thermal energy as the gas is compressed. This means, that when acting on a volume of gas, an amount of the output stroke’s energy is then converted to heat.
Delivering the solvent as a liquid incorporates further efficiency as the density (being the number of particles per unit volume) of gases, even under high pressure, is much lower than that of liquids. This means that two identical pumps, one primed with a volume of gaseous CO2, the other with an identical volume of liquid CO2, do not contain the same amount of solvent. The pump filled with the liquid CO2 contains more solvent molecules than the pump filled with gaseous CO2. This results in fewer pump strokes that are required to deliver a given amount of solvent when it is pumped as a liquid.
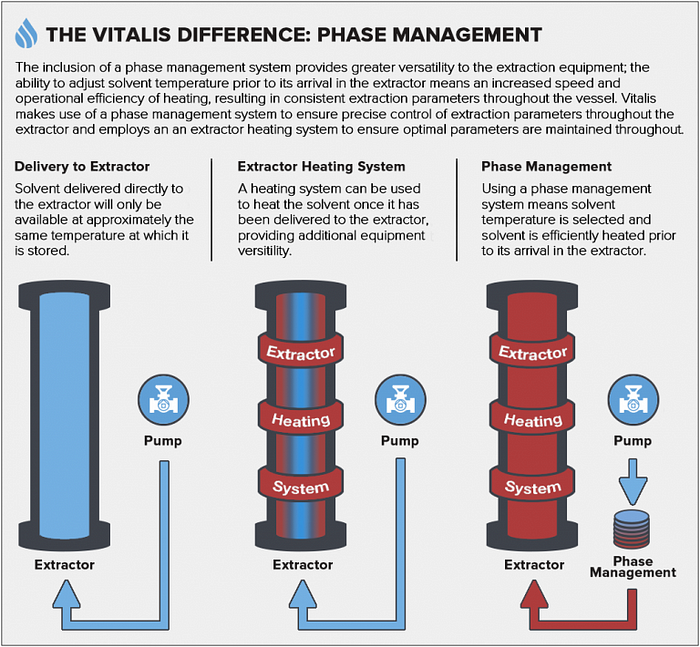
3. PHASE MANAGEMENT
Phase management is an optional stage during the extraction process. Temperature adjustments including those where a phase change is induced, can be made using a phase management system. To adjust the solvent temperature, the flow is directed through one or more coiled or folded solvent flow paths within heat-exchange bath(s) or vessel(s). These flow paths are designed to maximize the surface area and can be used to either increase or decrease solvent temperatures through the flow path piping.
From here CO2, either as a subcritical liquid or supercritical fluid, goes into the extraction chamber where the extraction process takes place, before following on to the separation stages. These stages will be covered in the ensuing two parts of this guided tour of a CO2 extraction system.
For more information visit us online at: https://vitaliset.com/